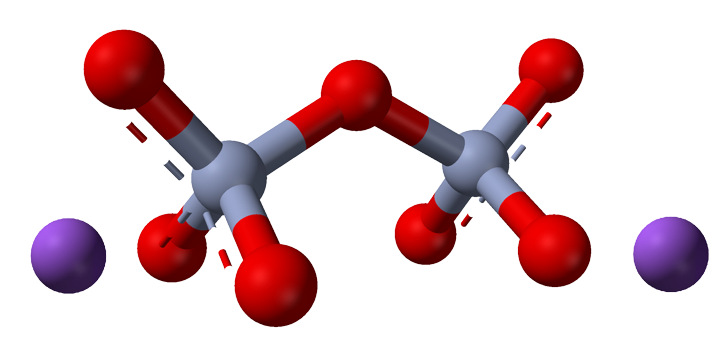
Sodium Dichromate
Sodium dichromate, with the chemical formula Na2Cr2O7, is a highly toxic and powerful oxidizing agent composed of sodium, chromium, and oxygen. It has several industrial applications but is known for its hazardous nature. Sodium dichromate is a chemically powerful but hazardous compound with various industrial applications, especially in the metal plating and chemical industries.
- Overview
- Application
- Spesification
Sodium dichromate (Na2Cr2O7) is a chemical compound with a bright orange-red color. It is a highly toxic and powerful oxidizing agent primarily composed of sodium, chromium, and oxygen. Here's an overview of sodium dichromate:
Chemical Composition: Sodium dichromate has the chemical formula Na2Cr2O7, which indicates that it consists of two sodium (Na) atoms, two chromium (Cr) atoms, and seven oxygen (O) atoms.
Physical Properties:
- Appearance: It typically appears as bright orange-red crystals or a fine powder.
- Solubility: Sodium dichromate is highly soluble in water, which makes it easily dissolved in aqueous solutions.
- Acidity: Its aqueous solution is acidic.
Key Characteristics and Uses:
- Oxidizing Agent: Sodium dichromate is a potent oxidizing agent, which means it has the ability to donate oxygen atoms or accept electrons from other substances, leading to chemical reactions.
- Chromium Plating: It is used in the electroplating industry to provide a protective and decorative chromium coating on metal surfaces. This process is commonly known as chromium plating.
- Wood Preservative: Historically, sodium dichromate has been used as a wood preservative to protect against decay and insects, but its use in this context has been significantly reduced due to environmental and health concerns.
- Chemical Production: It is used in the production of various chemicals and pigments, including those used in the textile, paint, and dye industries.
- Laboratory Reagent: Sodium dichromate is employed in chemical laboratories as an oxidizing agent and as a source of chromium in various chemical reactions and experiments.
- Analytical Chemistry: It serves as a reagent in analytical chemistry for conducting specific tests and analyses.
Hazards and Environmental Concerns:
- Toxicity: Sodium dichromate is highly toxic, both through ingestion and inhalation. It can cause severe health problems, including skin irritation, eye damage, respiratory issues, and is considered a carcinogen.
- Environmental Impact: It is considered hazardous waste, and its disposal is strictly regulated due to the risk of contaminating soil and water. Chromium contamination in water bodies can have detrimental ecological effects.
Regulations and Safety:
- Due to its extreme toxicity, there are strict regulations and safety guidelines governing the handling, storage, and disposal of sodium dichromate.
- Occupational safety measures, including the use of personal protective equipment (PPE), are essential when working with this compound.
Alternatives: In many applications, sodium dichromate has been replaced by less toxic and more environmentally friendly alternatives, such as trivalent chromium compounds, due to health and environmental risks associated with hexavalent chromium compounds like sodium dichromate.
Sodium dichromate (Na2Cr2O7) has several industrial and laboratory applications due to its strong oxidizing properties.
Here are some common applications of sodium dichromate:
Chromium Plating: Sodium dichromate is used in the electroplating industry to create a protective and decorative chromium layer on metal surfaces. Chromium plating enhances the appearance of objects and provides corrosion resistance.
Chemical Manufacturing: It is used in the production of various chemicals, including those used in the textile, paint, and dye industries. It can be an essential component in the synthesis of specific organic compounds.
Wood Preservative (Historical): In the past, sodium dichromate was used as a wood preservative to protect against decay and insect infestations. However, this application has been largely discontinued due to environmental and health concerns.
Analytical Chemistry: Sodium dichromate is employed as a powerful oxidizing agent in chemical laboratories for various analytical tests and experiments. It can be used to determine the concentration of reducing agents in solution, among other applications.
Laboratory Reagent: It serves as a reagent in laboratories for chemical reactions that require strong oxidizing conditions. Researchers use it to study oxidation-reduction reactions and other chemical processes.
Oxidation of Organic Compounds: Sodium dichromate can be used to oxidize organic compounds, transforming them into different products. This oxidation can be useful in various chemical syntheses.
Waste Treatment: In some wastewater treatment processes, sodium dichromate can be used to oxidize or break down certain organic pollutants and contaminants.
Photography (Historical): In traditional black and white photography, sodium dichromate was used in preparing light-sensitive emulsions on photographic plates and papers. This application has become less common with the advent of digital photography.
Below is a table summarizing the specifications of sodium dichromate (Na2Cr2O7):
| Property | Value |
|---|---|
| Chemical Formula | Na2Cr2O7 |
| Molecular Weight | Approximately 261.97 g/mol |
| Appearance | Bright orange-red crystals or powder |
| Solubility in Water | Highly soluble |
| Density | Approximately 2.35 g/cm³ at 20°C |
| Melting Point | Decomposes at approximately 400-600°C |
| Boiling Point | Decomposes at higher temperatures |
| pH of Aqueous Solution | Acidic |
| Oxidation State of Chromium | Hexavalent (Cr6+) |
| Hazardous Properties | Highly toxic, carcinogenic, corrosive |
| Odor | Odorless (but may have a faint metallic smell) |
Sodium dichromate is highly toxic and poses significant health and environmental risks. Handling, storage, and disposal must comply with strict safety and regulatory guidelines to minimize these risks.



